| dc.contributor.author | Sapkota, Dipak | en_US |
| dc.contributor.author | Bruland, Ove | en_US |
| dc.contributor.author | Parajuli, Himalaya | en_US |
| dc.contributor.author | Osman, Tarig Al-Hadi | en_US |
| dc.contributor.author | Teh, Muy-Teck | en_US |
| dc.contributor.author | Johannessen, Anne Christine | en_US |
| dc.contributor.author | Costea, Daniela Elena | en_US |
| dc.date.accessioned | 2016-03-22T10:20:34Z | |
| dc.date.available | 2016-03-22T10:20:34Z | |
| dc.date.issued | 2015-09-09 | |
| dc.Published | BMC Cancer 2015, 15:631 | eng |
| dc.identifier.issn | 1471-2407 | |
| dc.identifier.uri | https://hdl.handle.net/1956/11727 | |
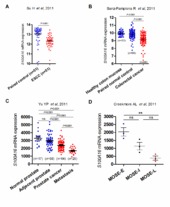
| dc.description.abstract | Background: Altered expression of S100A16 has been reported in human cancers, but its biological role in tumorigenesis is not fully understood. This study aimed to investigate the clinical significance and functional role of S100A16 in oral squamous cell carcinoma (OSCC) suppression. Methods: S100A16 mRNA and/or protein levels were examined by quantitative RT-PCR and immunohistochemistry in whole- and laser microdissected-specimens of normal human oral mucosa (NHOM, n = 65), oral dysplastic lesions (ODL, n = 21), OSCCs (n = 132) and positive cervical nodes (n = 17). S100A16 protein expression in OSCC was examined for correlations with clinicopathological variables and patient survival. S100A16 was over-expressed and knocked-down in OSCC-derived (CaLH3 and H357) cells by employing retroviral constructs to investigate its effects on cell proliferation, sphere formation and three dimensional (3D)-organotypic invasive abilities in vitro and tumorigenesis in a mouse xenograft model. Results: Both S100A16 mRNA and protein levels were found to be progressively down-regulated from NHOM to ODL and OSCC. Low S100A16 protein levels in OSCC significantly correlated with reduced 10-year overall survival and poor tumor differentiation. Analysis of two external OSCC microarray datasets showed a positive correlation between the mRNA expression levels of S100A16 and keratinocyte differentiation markers. CaLH3 and H357 cell fractions enriched for differentiated cells either by lack of adherence to collagen IV or FACS sorting for low p75NTR expression expressed significantly higher S100A16 mRNA levels than the subpopulations enriched for less differentiated cells. Corroborating these findings, retroviral mediated S100A16 over-expression and knock-down in CaLH3 and H357 cells led to respective up- and down-regulation of differentiation markers. In vitro functional studies showed significant reduction in cell proliferation, sphere formation and 3D-invasive abilities of CaLH3 and H357 cells upon S100A16 over-expression. These functional effects were associated with concomitant down-regulation of self-renewal (Bmi-1 and Oct 4A) and invasion related (MMP1 and MMP9) molecules. S100A16 over-expression also suppressed tumorigenesis of H357 cells in a mouse xenograft model and the resulting tumor xenografts displayed features/expression of increased differentiation and reduced proliferation/self-renewal. Conclusions: These results indicate that S100A16 is a differentiation promoting protein and might function as a tumor suppressor in OSCC. | en_US |
| dc.language.iso | eng | eng |
| dc.publisher | BioMed Central | eng |
| dc.rights | Attribution CC BY | eng |
| dc.rights.uri | http://creativecommons.org/licenses/by/4.0 | eng |
| dc.title | S100A16 promotes differentiation and contributes to a less aggressive tumor phenotype in oral squamous cell carcinoma | en_US |
| dc.type | Peer reviewed | |
| dc.type | Journal article | |
| dc.date.updated | 2015-11-10T11:24:53Z | |
| dc.description.version | publishedVersion | en_US |
| dc.rights.holder | Copyright 2015 The Authors | |
| dc.identifier.doi | https://doi.org/10.1186/s12885-015-1622-1 | |
| dc.identifier.cristin | 1284731 | |